Fluorophore, Mounting Media And Imaging Chamber Selection For Leica DM 6B Upright Microscope
Microscope Slides and Cover Glass Thickness
Most microscope objectives are designed to view the sample through a thin glass window, 0.17 mm thick (this is the #1.5 coverglass). This is the coverglass that you should use to get best resolution. The higher the numerical aperture (NA) of the dry objective, the more sensitive it is to proper coverglass thickness, NA is the number after the magnification of the objective (for instance, on a 10x/0.4 objective). On the DM6B microscope, objectives up to the 10x/0.32 are not very sensitive to coverglass thickness. However, with the 20x/0.55 objective, you will not get the best image if you do not use the correct coverglass or if you do not use coverglass at all.
The 40x/0.6 objective has almost the same resolution as the 20x/0.55, so for most thin samples, it is better to use the 20x objective and capture a larger field of view. The advantage of the 40x objective is its long working distance and the adjustable correction collar, that allows getting good quality images when imaging without coverglass or imaging through up to 2 mm layer glass (or other materials) IF ADJUSTED PROPERLY.
The 63x/0.6-1.4 oil immersion objective is not very sensitive to coverglass thickness, bacause the immersion oil has the same refractive index as the glass. This is the highest-resolution objective on this microscope. HOWEVER, this is only true when the adjustable aperture in the objective is fully open (NA = 1.4). Make sure to check the aperture settings on the objective if doing high resolution imaging. When the objective aperture is closed, the NA and thus the resolution is the same as on the 40x dry objective.
Recommended types of samples:
- The stage can accept regular Slides (25 x 75 mm) or oversized slides (up to 50 x 75 mm) with a 0.17 mm thick coverglass.
- If using a non-hardening mounting medium, the coverglass should be sealed along the edges with nail polish or other suitable sealer that has dried.
Fluorescence Microscopy, Fluorochromes
SUITABLE FLUOROCHROMES
If possible, avoid the traditional dyes like Fluorescein or FITC, They are not very bright and photobleach rapidly. use modern fluorochromes that are brighter and much more resistant to photobleaching. There are many brands/sources that work well, for instance Abberior, Atto, Alexa Fluor and Alexa Fluor Plus, CF dyes, cyanine dyes like Cy3 and Cy5, DyLight, Chromeo, AttoFluor, …
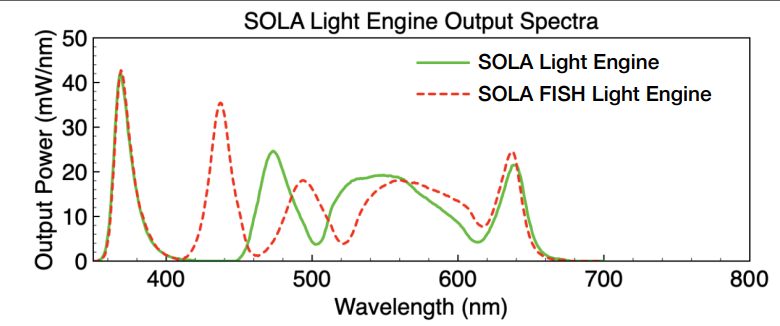
Remember, that the LED fluorescence illuminator (Lumencor SOLA) has a limited wavelength range (upper limit is around 630 nm), so it will not be useable with the near-infrared or infrared dyes that are excited in the 700 nm range.
MOUNTING MEDIA
Best image quality is achieved if the mounting medium has the same refractive index as the immersion medium for the objective (Water – 1.33, Glycerol – 1.47, Oil – 1.518). To reduce photobleaching of the fluorophores, mounting media may contain anti-fade reagents. Some fluorophores are not compatible with certain antifade reagents. Consult the mounting media datasheet for compatibility with fluorescent dyes. Here are some standard mounting media and their refractive indices:.
- 75% Glycerol – 1.44
- Citifluor 1.463
- Vectashield – 1.457
- Mowiol – 1.49
- ProLong Gold (hardening version) – Fresh mounting: 1.39 Cured for 1 day: 1.40 Cured for 4 days: 1.44
- Prolong Glass, Citifluor High Refractive index media, 2,2-Thiodiethanol (Staudt et al., 2007) – 1.515
- Live samples, or samples mounted in a buffer are best imaged with a water immersion objective. Using oil immersion objectives for these samples is NOT recommended, resolution and signal strength will degrade rapidly when focusing deeper into the sample.
- For routine imaging of fixed, fluorescently stained samples, non-hardening glycerol-based mounting media are convenient and work reasonably well with oil immersion objectives as well as glycerol immersion objectives. You can make your own media (see links to recipes below) or use commercially available products.
- FLUORESCENT PROTEINS require some water to retain their molecular structure. Thus, not all mounting media are suitable for such samples.
- Best resolution for samples in glycerol mountants is achieved using the 63x/1.3 glycerol-immersion objective.
- For highest resolution imaging with oil immersion objectives, the samples should be mounted in a medium that has the same refractive index as glass and immersion oil (1.515). If it is a hardening medium, it may shrink during curing and distort 3D samples, however. The 2,2- thiodiethanol is a suitable high-index medium, but is not compatible with fluorescent proteins at the optimal (97%) concentration.
For more information, see this paper: Ravikumar S, Surekha R, Thavarajah R. Mounting media: An overview. J NTR Univ Health Sci 2014;3, Suppl S1:1-8
RECIPES FOR ANTI-FADE MOUNTING MEDIA
- Medium with n-propyl gallate. From Jackson Immunoresearch website www.jacksonimmuno.com: “In our experience n-propyl gallate added to mounting media reduces fading of fluorescence from many different fluorophores during fluorescence microscopy. The following is a simple recipe for making an anti-fade mounting medium containing n-propyl gallate.”
• Prepare a 10X PBS stock solution.
• Prepare a stock solution of 20%(w/v) n-propyl gallate (Sigma P3130) in dimethyl formamide or dimethyl sulfoxide. (Note: n-propyl gallate does not dissolve well in water-based solutions.)
• Thoroughly mix 1 part of 10X PBS with 9 parts of glycerol (ACS grade 99-100% purity) and slowly add 0.1 part 20% n-propyl gallate dropwise with rapid stirring.
Store the medium in aliquots at -20 C.
2. Medium with p-phenylenediamine (from CSHL Protocols http://cshprotocols.cshlp.org/content/2019/2/pdb.rec104265.full?rss=1):
| Reagent | Amount to add | Final concentration |
|---|---|---|
| Tris-HCl (1 M, pH 8.8) | 0.8 mL | 20 mM |
| p-phenylenediamine | 0.20 g | 0.5% |
| Glycerol (100%) | 36 mL | 90% |
Mix the above ingredients together in a 50-mL conical tube. Adjust volume to 40 mL with H2O. Dissolve the solids by slowly bubbling nitrogen gas through a Pasteur pipette inserted deep into the mixture. Filter through a 0.8-µm filter at room temperature. Store at −20°C in 1- or 3-mL syringes devoid of air bubbles. Discard when dark brown. Handle this reagent with gloves; it causes stains when spilled.
